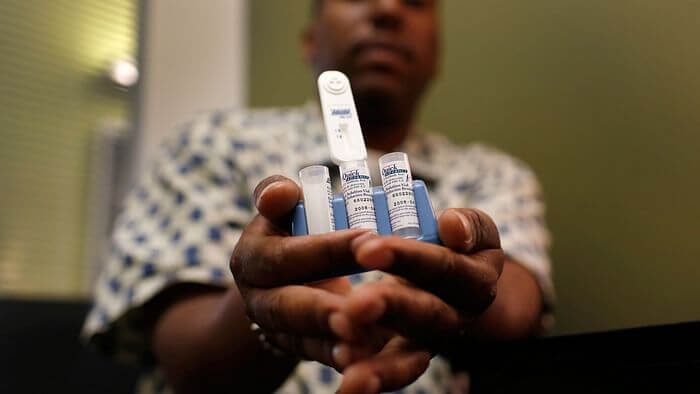
After a meeting between Indian Prime Minister Narendra Modi and the chief of the Indian Council of Medical Research (ICMR), Balram Bhargava, the Indian government announced its decision to cancel procurement orders for half a million testing kits from China. Further, the authorities decided to withdraw kits that are already in use from several states. Officials reassured local media houses that the cancellation will not cause the government any losses as it has yet to make any advance payment on the order.
The rapid testing kits are supposed to detect antibodies in the blood to identify COVID-19 positive patients, delivering results within thirty minutes. However, several states reported inaccuracies in the results of the tests. Raman R. Gangakhedkar, the head of epidemiology and communicable diseases at ICMR, said, “As we got complaints from some states about the inaccuracy of the test results, we spoke with two more states and found major variations. In some places, it is 6% while in others it is 71%.”
The issue was further complicated after the Delhi High Court criticised the ICMR for procuring kits at a higher price. In the order, Justice Najmi Waziri slashed the price of the kits by 33%, from Rs. 600 per test to Rs. 400.
In response to the allegations, the Spokesperson and Counselor of the Chinese Embassy in India, Ji Rong, issued a statement saying, “The quality of medical products exported from China is prioritised. It is unfair and irresponsible for certain individuals to label Chinese products as ‘faulty’ and look at issues with pre-emptive prejudice.” He added that the inaccuracies could be the result of agencies mishandling the kits and failing to carry out the tests in adherence to the strict requirements for storage, transportation, and use.
In March, the Netherlands recalled roughly 600,00 masks from China out of a shipment of 1.3 million after an inspection determined that they fail to adequately protect the face or have defective filters. Spain returned 640,000 rapid testing kits produced by Chinese company Bioeasy as they had an accuracy level of below 30%. Czech Republic said that the rapid test kits that it bought from China are not working as intended. Turkey’s Health Minister also said that the samples of rapid testing kits it had received from a Chinese company did not meet the country’s standards, and thus it would be suspending its order. And The UK government is seeking a refund for millions of fingerprick coronavirus tests ordered from China after a trial by Oxford University found them to be unreliable.
Image Source: The Quint
India Cancels Order for Half a Million Rapid Testing Kits From China
Several states reported inaccuracies in the results of the tests.
April 28, 2020